Update Chrome Browser
Got itchy feet? Earn $1,850
Help us advance scientific research and earn $1,850 for your child by signing up for our Athlete's foot study today!
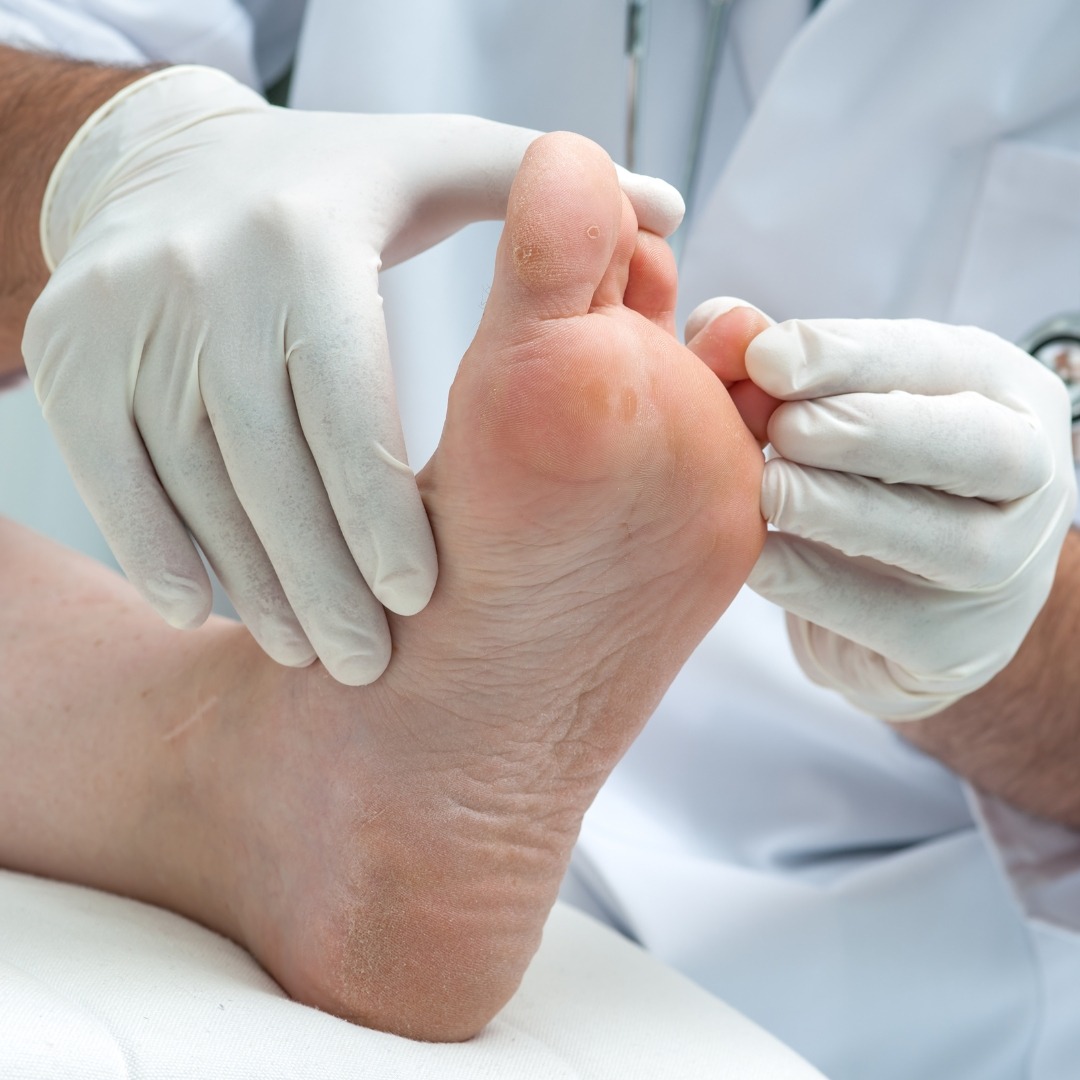
Athletes Foot- Tinea Pedis
What’s This Study About?
We’re studying a new way of applying a marketed product as a foot spray designed to tackle athlete’s foot (that annoying, itchy skin between your toes). This study is about ensuring it works as intended. The study is approved by the FDA.
Who Can Join?
Anyone with athlete’s foot aged 9 and above can sign up, but we’re particularly seeking kids and teenagers aged 9-17.
What Will My Son/Daughter Need to Do?
- Must have redness, itching, stinging, cracking, peeling between the toes
- Have a physical exam by the physician
- Our medical staff will apply the foot spray
- Must attend 12 visits over 6 weeks to our clinic
- Be willing to have blood drawn
- Must be accompanied by a parent/legal guardian
By participating in this study, you can earn up to $1,850.
Please feel free to call us with any safety concerns or questions.
Menu
Copyright © 2024 DFW Clinical Research | Digital Marketing by Estrada Marketing
